Diagnosis and treatment of Malaria
Prompt and accurate diagnosis is critical to the effective management of malaria. A malaria infection can be detected through clinical diagnostics or laboratory diagnostics but because of the non-specific nature of the signs and symptoms of malaria, it is advisable to combine the two types of diagnostic tests (Tangpukdee et al., 2009).
Clinical diagnosis of malaria is based on the patients’ signs and symptoms, and on physical findings at examination. In the laboratory, malaria is diagnosed using different techniques.
The gold standard for laboratory diagnosis is the microscopic examination of stained blood films using Giemsa, Wright’s, or Field’s stains (Warhurst and Williams, 1996; Bharti et al., 2007).
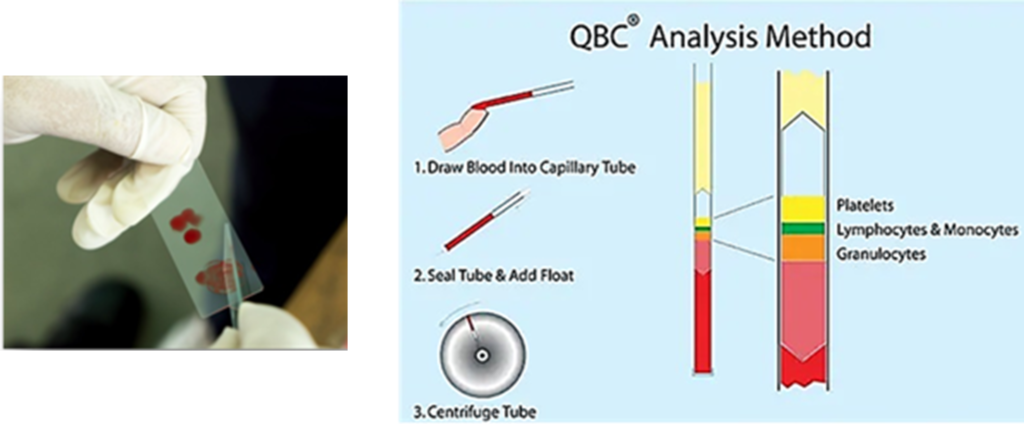
Another technique, the QBC technique, provides staining the Plasmodium genome in microhematocrit tubes with fluorescent dyes, such as acridine orange, and its subsequent detection by epifluorscent microscopy. Parasite nuclei appear bright green, instead cytoplasm yellow-orange (Chotivanich et al., 2006).
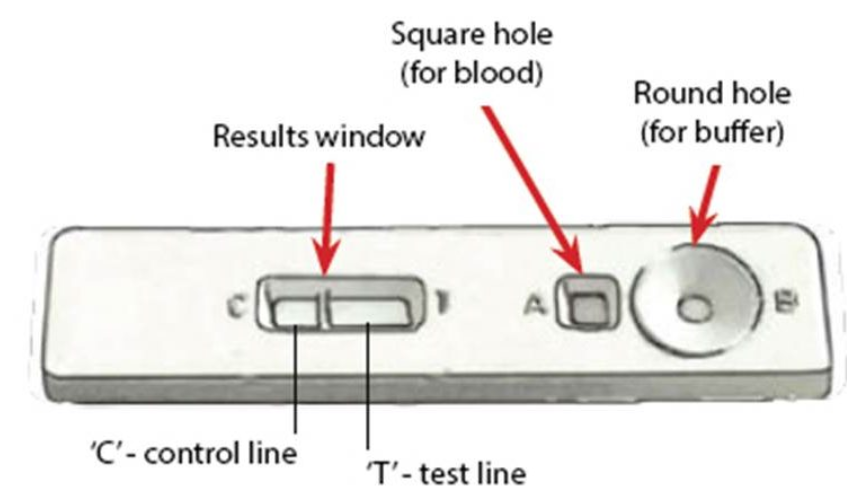
Rapid tests for the diagnosis of malaria (RTDs) are also available, e.g. OptiMAL (Tagbor et al., 2008; Zerpa et al., 2008), ICT (Ratsimbasoa et al., 2008), Para-HIT-f (McMorrow et al., 2008), ParaScreen (Endeshaw et al., 2008), SD Bioline (Lee et al., 2008), Paracheck (Harvey et al., 2008). RDTs are all based on the same principle and detect malaria antigen (a P. falciparum-specific protein, e.g. histidine-rich protein II, lactate dehydrogenase, aldolase, etc…) in blood flowing along a membrane containing specific anti-malaria antibodies.
Diagnostic techniques for malaria based on molecular analysis have recently been developed. They display a higher sensitivity and a higher specificity compared to traditional diagnostic techniques. The most common are PCR, loop-mediated isothermal amplification (LAMP), microarray, mass spectrometry (MS), and flow cytometric (FCM) assay techniques.
Regarding the FCM, the principle of this technique is based on detection of hemozoin, which is produced when the intra-erythrocytic malaria parasites digest host hemoglobin and crystallize the released toxic heme into hemozoin in the acidic vacuole. Hemozoin within phagocytotes can be detected by depolarization of laser light, as cells pass through a flow-cytometer channel. (Wongchotigul et al., 2004; Shapiro and Mandy, 2007; Izumiyama et al., 2009)
Once the diagnosis of malaria has been made, appropriate antimalarial treatment must be initiated immediately.
Treatment of malaria depends on many factors including:
- The species of malaria parasite that has caused the disease;
- Clinical status of the patient;
- Expected drug susceptibility of the infecting parasite as determined by the geographic area where the infection has acquired;
- Previous use of antimalarials, including those taken for malaria chemoprophylaxis.
The most common antimalarial drugs include:
- Chloroquine phosphate. Chloroquine phosphate is the preferred treatment if the infection is considered uncomplicated and is caused by any parasite of the species P. falciparum that is sensitive to the drug. It concentrates within the parasite acid vesicles, raising internal pH and so inhibiting parasite growth (Naß and Efferth, 2018; Pinheiro et al., 2018).
- Hydroxychloroquine. Hydroxychloroquine is also an acceptable first-line treatment of chloroquine-sensitive P. falciparum and has a similar mechanism of action as chloroquine phosphate (Naß and Efferth, 2018; Pinheiro et al., 2018).
- Primaquine phosphate. Primaquine phosphate is added to either chloroquine phosphate or hydroxychloroquine when infections are caused by P. vivax or P. ovale with chloroquine sensitivity. This medication works by eliminating the parasite that remain dormant in the patient’s liver (Karunajeewa and James, 2020).
- Atovaquone-proguanil. Atovaquone-proguanil is utilized in infections caused by P. falciparum with chloroquine resistance. Atovaquone selectively inhibits parasite mitochondrial electron transport, and proguanil inhibits dihydrofolate reductase disrupting deoxythymidylate synthesis (Hill et al., 2022).
- Artemether-lumefantrine. Artemether-lumefantrine is an alternative first-line option for the treatment of malaria caused by chloroquine-resistant P. falciparum. The mechanism of action is to inhibit nucleic acid and protein synthesis (Hamaluba et al., 2021).
- Quinine sulfate plus doxycycline, tetracycline, or clindamycin. Quinine sulfate plus doxycycline, tetracycline, or clindamycin is a second-line option for treating malaria caused by chloroquine-resistant P. falciparum. Quinine intercalates into DNA, disrupting the parasites’ replication and transcription (Hill et al., 2022).
- Quinidine gluconate. Quinidine gluconate is the drug of choice for severe malaria because it is the only parenterally available antimalarial drug (Hill et al., 2022).
Sources:
1. Bharti, A.R., et al. (2007) Polymerase chain reaction detection of Plasmodium vivax and Plasmodium falciparum DNA from stored serum samples: implications for retrospective diagnosis of malaria. Am J Trop Med Hyg. 77, 444-6
2. Chotivanich, K., et al. Laboratory diagnosis of malaria infection-a short review of methods. (2006) Aust J Med Sci. 27, 11-5
3. Endeshaw, T., et al. (2008) Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 7, 118
4. Hamaluba, M., et al. (2021) Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a single-centre, open-label, randomised, non-inferiority trial. Lancet Infect Dis. 21, 1395-1406
5. Harvey, S.A., et al. (2008) Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J. 7, 160
6. Hill, S.R., et al. (2022) Antimalarial Medications In: Hill, S.R., Thakur, R.K., Sharma, G.R. Editors: StatPearls. Treasure Island, FL
7. Izumiyama, S., et al. (2009) Plasmodium falciparum: development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Exp Parasitol. 121, 144-50
8. Karunajeewa, H., James, R. (2020) Primaquine for Plasmodium vivax malaria treatment. Lancet. 395, 1971-2
9. Lee, S.W., et al. (2008) Rapid diagnosis of vivax malaria by the SD Bioline Malaria Antigen test when thrombocytopenia is present. J Clin Microbiol. 46, 939-42
10. McMorrow, M.L., et al. (2008) Challenges in routine implementation and quality control of rapid diagnostic tests for malaria-Rufiji District, Tanzania. Am J Trop Med Hyg. 79, 385-90
11. Naß, J., Efferth, T. (2018) The activity of Artemisia spp. and their constituents against Trypanosomiasis. Phytomedicine. 47, 184-91
12. Pinheiro, L.C.S., et al. (2018) Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives. An Acad Bras Cienc. 90, 1251-71
13. Ratsimbasoa, A., et al. (2008) Evaluation of two new immunochromatographic assays for diagnosis of malaria. Am J Trop Med Hyg. 79, 670-2
14. Shapiro, H.M., Mandy, F. (2007) Cytometry in malaria: moving beyond Giemsa. Cytometry A. 71, 643-5
15. Tagbor, H., et al. (2008) Performance of the OptiMAL dipstick in the diagnosis of malaria infection in pregnancy. Ther Clin Risk Manag. 4, 631-6
16. Tangpukdee, N., et al. (2009) Malaria Diagnosis: A Brief Review. Korean J Parasitol. 47, 93-102
17. Warhurst, D.C., Williams, J.E. (1996) Laboratory diagnosis of malaria. J Clin Pathol. 49, 533-8
18. Wongchotigul, V., et al. (2004) The use of flow cytometry as a diagnostic test for malaria parasites. Southeast Asian J Trop Med Public Health. 35, 552-9
19. Zerpa, N., et al. (2008) Evaluation of the OptiMAL test for diagnosis of malaria in Venezuela. Invest Clin. 49, 93-101
· www.cdc.gov
· www.yourgenome.org