Malaria in pregnancy
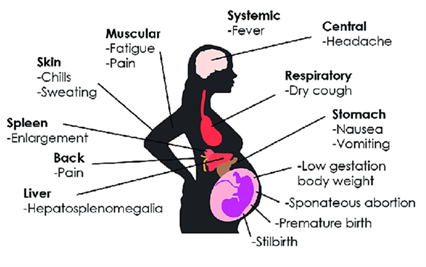
Pregnant women and children up to 5 years are the categories most at risk of infection and complications from malaria.
In 2015, an estimated 438,000 malaria deaths around the world have been reported, of which approximately 69% were children under 5 years of age. Of all malaria deaths, 90% were reported from African regions and rest were from South-East Asia region and the Eastern Mediterranean region. In malaria endemic areas, it is estimated that at least 25% of pregnant women are infected with malaria which causes 10,000 maternal and 200,000 neonatal deaths per year globally (Schantz-Dunn and Nour, 2009).
Regarding pregnancy, malaria is dangerous to both, the pregnant mother, and the developing fetus (Saito et al., 2020) .
Malaria tends to be more severe in pregnant women compared to non-pregnant population. This seems to be due to the hormonal, immunological and hematological changes of pregnancy, and the presence of the placenta. During pregnancy, cell-mediated immune response is halted which is required to support the development of placenta and the fetus. The general immune suppression during pregnancy makes women more susceptible to many infections (including malaria), especially during first trimester, and the low cell-mediated immune response in placenta makes it preferred site for the parasite of malaria to hide from host immune responses (Sharma and Shukla, 2017).
In fact, Plasmodium falciparum is able to accumulate in the placenta in a very specific way (P. vivax does not sequester in the placenta)(McGready et al., 2004). Scientific studies have shown that, in vitro, erythrocytes infected by malaria parasites can adhere to chondroitin sulphate A and hyaluronic acid, two receptors expressed on vascular endothelial cells of the placental system, and sequester in the placenta (Rogerson et al., 2007).
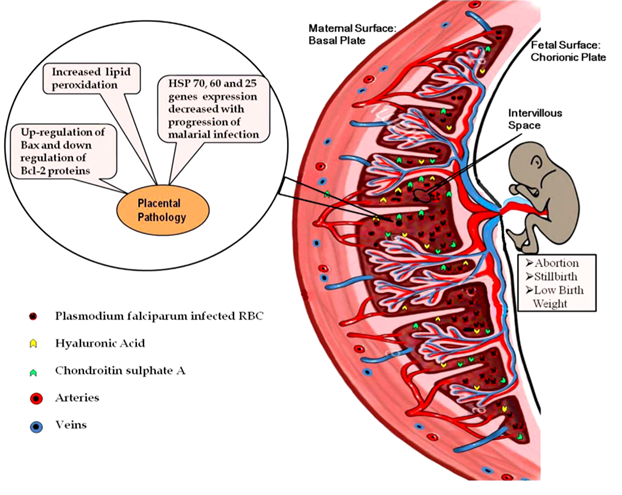
The principal parasite ligand, mediating chondroitin sulphate A adhesion and placental sequestration, is a specific surface antigen known as VAR2CSA protein (Salanti et al., 2003; Duffy et al., 2005).
However, as malarial infection progresses, there is an increase in cell-mediated immune response and an increased synthesis of pro-inflammatory cytokines, such as TNF, interleukin 2, and interferon γ to counteract the parasite in the local placental environment. This results in a massive recruitment of macrophages and T-cells to intervillous spaces of placenta. Increase in immune response is deleterious because causes oxidative stress and apoptotic cell death in placenta (Clark et al., 1986; Ockenhouse and Shear, 1984; Wozencraft et al., 1984; Sharma et al., 2012).
The placental insufficiency to provide nutrients to the fetus has, as consequences, intra uterine growth retardation, low birth weight, premature delivery and abortions.
Malarial infection in pregnant women instead results into clinical complications, such as anemia, pulmonary edema, hypoglycemia, cerebral malaria, puerperal sepsis, and some time death too (Menendez et al., 2000; Watkinson and Rushton, 1983).
Prevention
In areas with low transmission, control of P. falciparum malaria in pregnancy mostly depends on early detection by screening asymptomatic women for malaria at the first antenatal visit, passive case detection of symptomatic cases, and insecticide treated nets given as part of antenatal care (Desai et al., 2018).
In areas of moderate to high P. falciparum transmission, instead, preventing malaria in pregnancy relies on two combined approaches. First is represented by insecticide treated nets and the second one is the intermittent preventive treatment in pregnancy (IPTp) using the antimalarial sulphadoxine-pyrimethamine (SP) at predetermined intervals.
The most updated WHO malaria guidelines (March 2023) provide for the use of at least three doses of IPTp-SP during pregnancy. The drug combination is administered in the formulation of three tablets (each tablet containing 500 mg/25 mg SP), for the total required dosage of 1500 mg/75 mg SP. IPTp-SP should start in the second trimester (not before week 13 of pregnancy) and doses should be given at each scheduled antenatal care (ANC) contact until the time of delivery, provided that doses are at least one month apart.
Unfortunately, a large number of women have developed resistance to SP (particularly in east and southern Africa) and therefore its effectiveness has decreased. Resistance to SP arises through different single nucleotide polymorphisms in the genes encoding dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS), the targets of pyrimethamine and sulfadoxine, respectively (Rogerson et al., 2007; Van Eijk, 2019). These decrease parasite drug sensitivity to a degree that depends on the number and pattern of mutations (White, 2005).
In addition to the problem of resistance to the combination of drugs, there are also other kind of issues (socio-cultural, individual, environmental, sanitary) that discourage taking them.
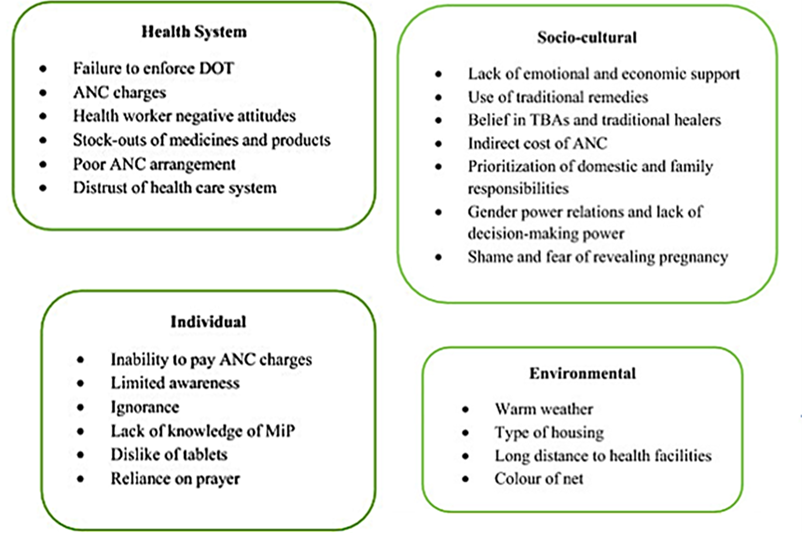
Health system factors such as failure to enforce directly observed therapies, charges for ANC and IPTp-SP services, negative attitudes of maternal health providers, stock-outs and poor ANC arrangements, failure to educate women about the benefits of SP and shortage of health staff, demotivated pregnant women from accessing ANC and to complete the IPTp-SP treatment (Anders et al., 2008; Hill et al., 2013; Thiam et al., 2013; Pell et al., 2011; Olaleye and Walker, 2020).
Individual factors such as difficulty in swallowing the tablets, potential side effects of the drugs, difficulty to pay ANC charges, and ignorance, discourage the intake of IPTp-SP (Nyaaba et al., 2021; Aberese-Ako et al., 2020; Aberese-Ako et al., 2021).
Socio-cultural factors such as the shame of being pregnant out of wedlock or being pregnant at the same time as their mothers, the poverty, lack of permission from their husbands and male heads of families to attend ANC, use of traditional and ancient remedies, result in non-participation or irregular participation in the ANC (Klein et al., 2016; Hill et al., 2015).
Environmental factors such aswarm weather and long distance to health facilities especially for women who live in rural areas demotivated them from accessing IPTp-SP (Azizi et al., 2018; Bhutta et al., 2009).
Source:
1. Aberese-Ako M., et al. (2022) Motivators and demotivators to accessing malaria in pregnancy interventions in sub-Saharan Africa: a meta-ethnographic review. Malar J. 21, 170
2. Aberese-Ako, M., et al. (2021) An ethnographic study of how health system, socio-cultural and individual factors influence uptake of intermittent preventive treatment of malaria in pregnancy with sulfadoxine-pyrimethamine in a Ghanaian context. PLoS ONE. 16
3. Aberese-Ako, M., et al. (2020) Managing intermittent preventive treatment of malaria in pregnancy challenges: an ethnographic study of two Ghanaian administrative regions. Malar J. 19, 347
4. Anders, K., et al. (2008) Timing of intermittent preventive treatment for malaria during pregnancy and the implications of current policy on early uptake in north-east Tanzania. Malar J. 7, 79
5. Azizi, S.C., et al. (2018) Uptake of intermittent preventive treatment for malaria during pregnancy with sulphadoxine-pyrimethamine (IPTp-SP) among postpartum women in Zomba District, Malawi: a cross-sectional study. BMC Pregnancy Childbirth. 18, 108
6. Bhutta, Z.A., et al. (2009) Delivering interventions to reduce the global burden of stillbirths: improving service supply and community demand. BMC Pregnancy Childbirth. 9
7. Clark, I.A., et al. (1986) Oxygen-derived free radicals in the pathogenesis of parasitic disease. Adv Parasitol. 25, 1-45
8. Desai, M., et al. (2018) Prevention of malaria in pregnancy. Lancet Infect Dis. 18
9. Hill, J., et al. (2013) Factors affecting the delivery, access, and use of interventions to prevent malaria in pregnancy in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 10
10. Hill, J., et al. (2015) Access and use of interventions to prevent and treat malaria among pregnant women in Kenya and Mali: a qualitative study. PLoS ONE. 10
11. Klein, M.C., et al. (2016) “There is no free here, you have to pay”: actual and perceived costs as barriers to intermittent preventive treatment of malaria in pregnancy in Mali. Malar J. 15, 158
12. McGready, R., et al. (2004) The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 70, 398-407
13. Menendez, C., et al. (2000) The impact of placental malaria on gestational age and birth weight. J Infect Dis. 181,1740-5.
14. Nyaaba, G.N., et al. (2021) A socio-ecological approach to understanding the factors influencing the uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in South-Western Nigeria. PLoS ONE. 16
15. Ockenhouse, C.F., Shear, H.L. (1984) Oxidative killing of the intra erythrocytic malaria parasite P. yoelii by activated macrophages. J Immunol. 132, 424-31
16. Olaleye, A.O., Walker, O. (2020) Impact of health systems on the implementation of intermittent preventive treatment for malaria in pregnancy in sub-Saharan Africa: a narrative synthesis. Trop Med Infect Dis. 5, 134
17. Pell, C., et al. (2011) Social and cultural factors affecting uptake of interventions for malaria in pregnancy in Africa: a systematic review of the qualitative research. PLoS ONE. 6
18. Rogerson, S.J., et al. (2007) Malaria in Pregnancy: Linking Immunity and Pathogenesis to Prevention. Am J Trop Med Hyg. 77, 14-22
19. Schantz-Dunn, J., Nour, N.M. (2009) Malaria and pregnancy: a global health perspective. Rev Obstet Gynecol. 2, 186-92
20. Sharma, L., et al. (2012) Role of oxidative stress and apoptosis in the placental pathology of Plasmodium berghei infected mice. PLoS One. 7
21. Sharma, L., Shukla, G. (2017) Placental Malaria: A new insight into the Pathophysiology. Front Med (Lausanne). 4, 117
22. Thiam, S., et al. (2013) Why are IPTp coverage targets so elusive in sub-Saharan Africa? A systematic review of health system barriers. Malar J. 12, 353
23. Van Eijk, A. M., et al. (2019) Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infectious Diseases. 19, 546-56
24. Watkinson, M., Rushton, D.I. (1983) Plasmodial pigmentation of placenta and outcome of pregnancy in West African mothers. Br Med J.287, 251-4
25. White, N.J. (2005) Intermittent presumptive treatment for malaria. PLoS Med. 2.
26. Wozencraft, A.O., et al. (1984) Killing of human malaria parasite by macrophage secretory products. Infect Immun. 43, 664-9
· www.who.int