Prevention of Malaria

Antimalarial drugs can also be used to do chemoprophylaxis, so to prevent malaria.
Another effective option for prevention is to keep the vector of the disease under control, using different strategies:
–Indoor residual spraying consists on spraying insecticide, once or twice a year, on all indoor surfaces where mosquitoes are likely to rest.
–Long-lasting insecticidal nets are mosquito nets that are also sprayed with an insecticide. They provide a physical and chemical barrier to the mosquito vector at night when the mosquito is most likely to bite.
–Larviciding is done by treating the breeding sites of the mosquito with substances that kill the larval stages of the insect.
In addition to chemoprophylaxis and to the strategies that keep the vector of malaria under control, immunoprophylaxis is also a valid alternative.
RTS,S/AS01 (commercial name Mosquirix) is a recombinant protein-based malaria vaccine, effective against Plasmodium falciparum. The vaccine’s use requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1-2 years. Its effectiveness is between 26% and 50%.
It was first developed in 1986 but its large-scale use was only approved by WHO in October 2021.
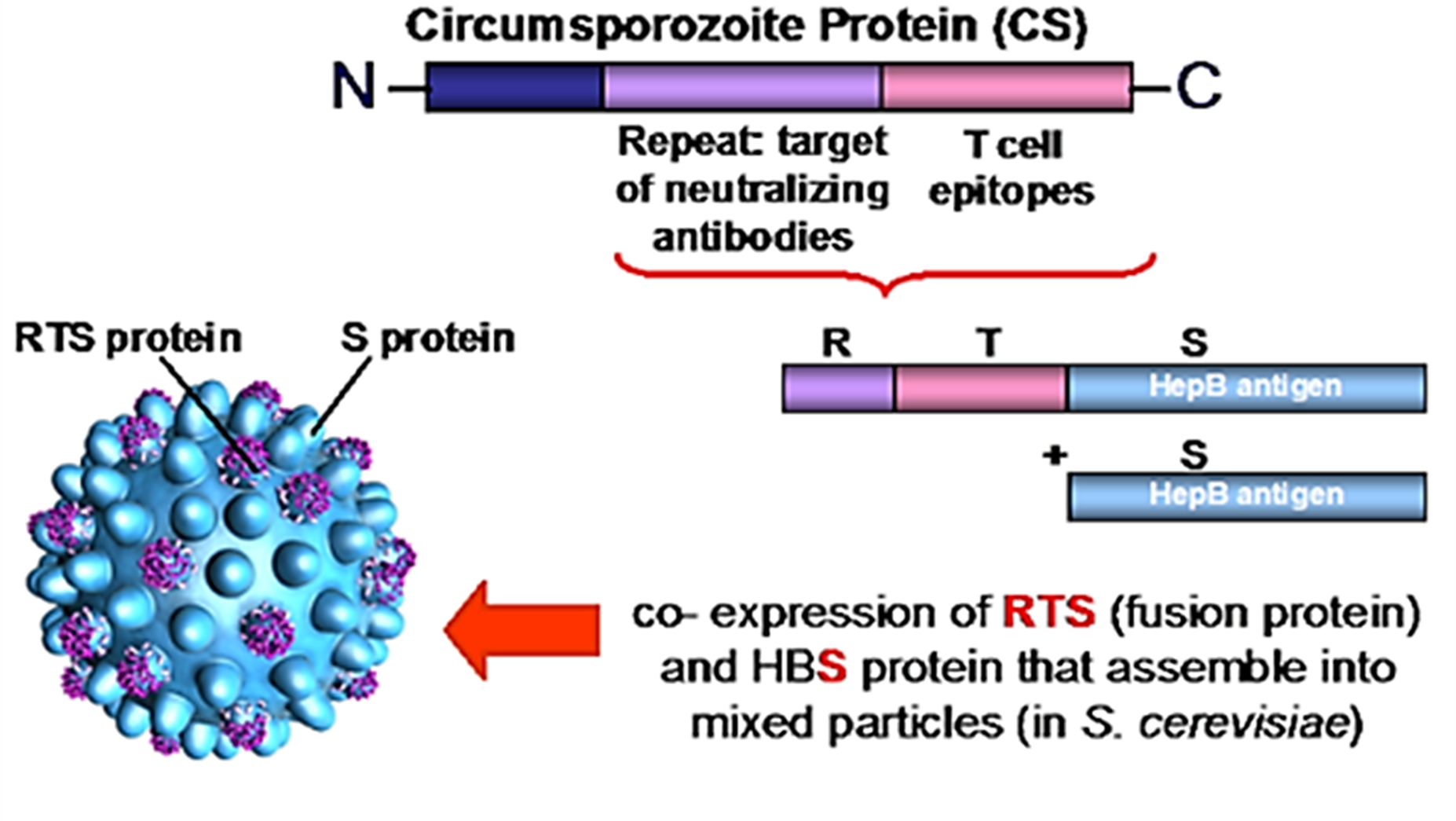
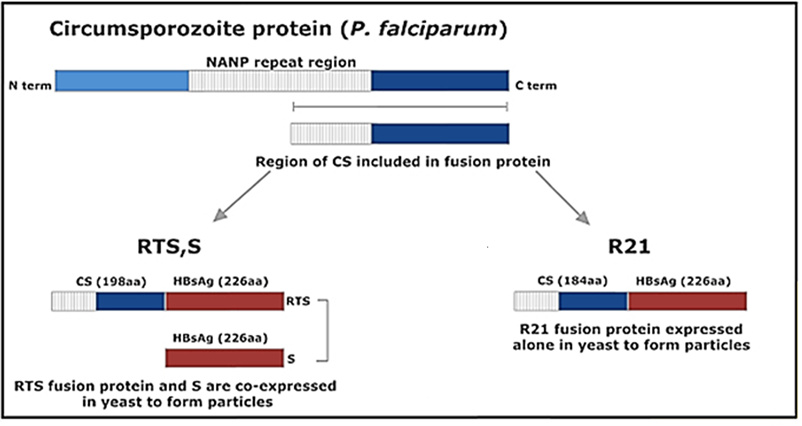
Vaccine’s name (RTS,S) derived from the genes used for the engineering:
1) “R”-> represents the central repeat region of the pre-erythrocytic circumsporozoite protein, a single polypeptide chain corresponding to a highly-conserved tandem repeat tetrapeptide NANP amino acid sequence.
2) “T” -> represents T-lymphocyte epitopes.
3) “S” -> indicates the gene encoding for the portion of Hepatitis B surface antigen (HBsAg)
The RTS genes are included in a first construct and are co-expressed in the yeast Saccharomyces cerevisiae with a second gene, encoding again for the portion of Hepatitis B surface antigen (hence the extra “S”), presents in a second construct (Laurens, 2020).
Soluble virus-like particles similar to the outer shell of a hepatitis B virus derive from the co-expression of the two constructs in yeast (Rutgers et al., 1988). Because of co-expression, each particle exposes the RTS fusion protein and the HBsAg on its surface.
A chemical adjuvant (AS01) is added to the formulation to increase the immune system response.
A new generation RTS,S-like vaccine, called R21, has also been developed in the last few years. In this case, a single construct containing the R, T and S genes, is expressed in Saccharomyces cerevisiae. In addition, Matrix-M is used as adjuvant instead of AS01 in this new vaccine (Collins et al., 2017).
Phase 2 data, related to R21, was published in September showing high effectiveness following a fourth booster dose, while the phase 3 data is expected to be published by the end of 2023.
Ghana has become the first country in the world to approve R21 malaria vaccine for use in children aged 5 to 36 months, who are at highest risk of death from malaria.
WHO is currently assessing whether to prequalify the vaccine for wider use.
Keywords:
prevention, Indoor residual spraying, long-lasting insecticidal nets, virus-like particles, RTS,S, R21, Saccharomyces cerevisiae, Hepatitis B surface antigen.
Source:
1. Collins, K.A., et al. (2017) Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep. 7, 46621
2. Laurens M.B. (2020) RTS,S/AS01 vaccine (Mosquirix™): an overview. Hum Vaccin Immunother. 16, 480-9
3. Rutgers, T., et al. (1988) Hepatitis B Surface Antigen as Carrier Matrix for the Repetitive Epitope of the Circumsporozoite Protein of Plasmodium Falciparum. Nature Biotechnology. 6, 1065-70
· www.gavi.org
· www.yourgenome.org